Quick Takeaways
-
Novel Compound Synthesis: Researchers at OIST have synthesized a stable 20-electron ferrocene derivative, challenging the century-old 18-electron rule in organometallic chemistry.
-
Redox Properties: This breakthrough introduces unconventional redox properties, allowing access to new oxidation states and enhancing the potential for applications in catalysis and materials science.
-
Implications for Sustainable Chemistry: Insights from this research can aid in designing tailor-made molecules with applications in sustainable chemistry, green catalysts, and next-generation materials.
- Future Innovation Platform: The study expands the conceptual toolkit for chemists, opening avenues for new technologies and applications in fields like energy storage, pharmaceuticals, and advanced catalysts.
Revising Chemical Principals
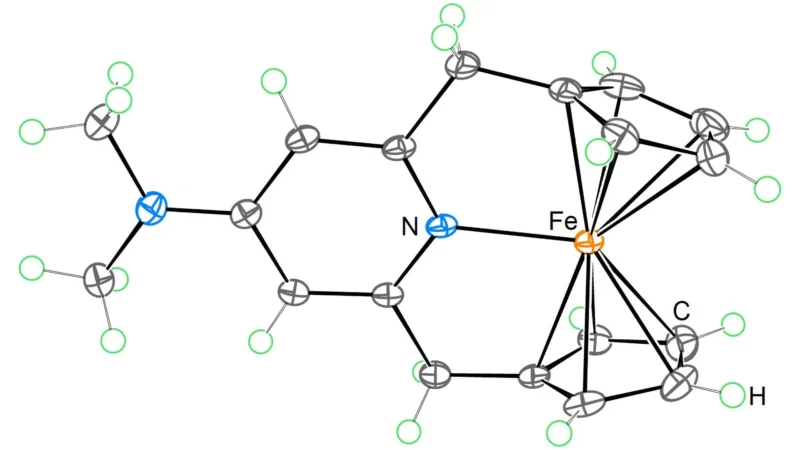
For over a century, the 18-electron rule has dominated our understanding of organometallic chemistry. This rule suggests that transition metal complexes achieve maximum stability with 18 valence electrons. However, researchers at the Okinawa Institute of Science and Technology have shattered this long-standing belief. They synthesized a stable 20-electron derivative of ferrocene, an iron-based complex. This achievement not only challenges chemical norms but also opens avenues for new scientific exploration.
The new compound demonstrates how adding two more electrons can enhance redox properties. Initially, ferrocene served as a pivotal discovery, earning a Nobel Prize in Chemistry for altering our grasp of metal-organic bonding. The OIST team built upon this legacy, crafting a ligand system that enables the coexistence of stability and additional electrons. Now, chemists can access unconventional oxidation states that could transform its utility in catalysis, materials science, and energy storage.
A Catalyst for Future Opportunities
Ferrocene derivatives have already found diverse applications, from solar cells to pharmaceuticals. This breakthrough provides chemists a fresh perspective on molecular design. It enhances the potential for creating green catalysts and next-generation materials.
The Organometallic Chemistry Group at OIST focuses on uncovering the principles that govern metal-organic interactions. Their work symbolizes a new frontier in chemical innovation, emphasizing the importance of challenging established rules. Ultimately, this synthesis not only enriches our understanding of chemistry but also lays the groundwork for sustainable solutions in various fields. The implications of this research resonate far beyond the lab, inviting a future filled with new possibilities.
Stay Ahead with the Latest Tech Trends
Stay informed on the revolutionary breakthroughs in Quantum Computing research.
Discover archived knowledge and digital history on the Internet Archive.
TechV1