Fast Facts
-
Breakthrough Treatment: ENCELTO, a neuroprotective surgical implant, has shown significant potential in slowing vision loss in people with macular telangiectasia type 2 (MacTel), marking the first approved treatment for this orphan retinal disorder.
-
Clinical Evidence: Results from two phase 3 clinical trials, involving 228 participants across 47 sites, demonstrated that ENCELTO significantly reduced the degeneration of light-sensing retinal cells by up to 54.8%, confirming its efficacy.
-
Mechanism of Action: The device continuously releases ciliary neurotrophic factor (CNTF), a protein known to protect retinal neurons, housed in a protective capsule that prevents immune rejection, allowing for long-term localized therapy.
-
Future Implications: The success of ENCELTO may pave the way for similar treatments targeting other neurodegenerative diseases, as the approach of sustained protein delivery could be adapted beyond MacTel.
A Breakthrough in Vision Preservation
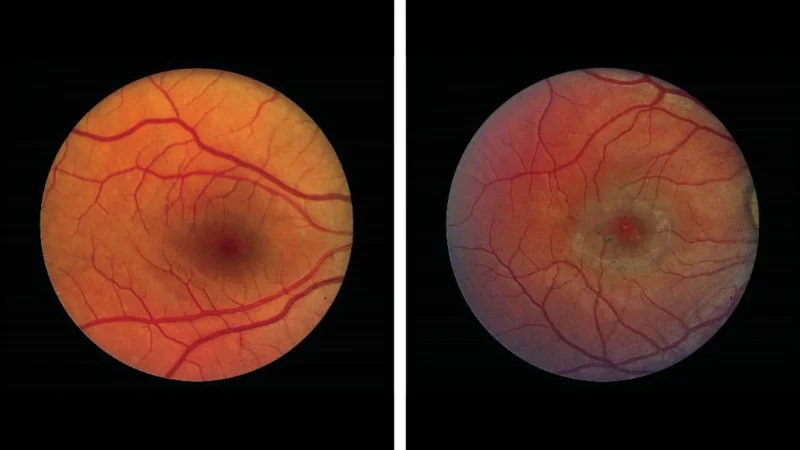
The FDA’s approval of the tiny eye implant ENCELTO marks a transformative moment for people suffering from macular telangiectasia type 2 (MacTel), a rare retinal disorder that leads to severe vision loss. For years, patients had few options, leading to despair as their central vision deteriorated. The groundbreaking study by researchers at Scripps Research and NIH demonstrates that this surgical implant can significantly slow down the loss of light-sensing retinal cells.
Through two phase 3 clinical trials involving 228 participants, the results showed promising outcomes. One trial reported a 54.8% reduction in retinal cell degeneration, while the other demonstrated a 30.6% reduction. Such data challenge the traditional understanding of treating eye diseases. Instead of merely accepting vision loss, this therapy offers a proactive approach. By continuously releasing a neuroprotective protein directly into the retina, ENCELTO aims to preserve vision rather than allowing it to fade away.
The Path Forward for Widespread Adoption
Despite the exciting results, the road to widespread adoption remains nuanced. The variability in trial outcomes highlights the complexity of measuring functional vision, especially in a slowly progressing disease like MacTel. Although some visual function measures appeared inconclusive, the overall trend supports the implant’s effectiveness—especially for patients who begin treatment early.
Patients tolerated the procedure well, experiencing minimal side effects. This opens the door for broader applications beyond MacTel, as the technology’s principles may extend to other neurodegenerative diseases. As researchers continue to refine treatment methods, they can tailor interventions to maximize patient benefits. Ultimately, ENCELTO represents not just a new product but a vital advancement in the human journey to preserve sight and enhance quality of life. The future holds promise for many who have long awaited effective therapies for rare conditions like MacTel.
Expand Your Tech Knowledge
Learn how the Internet of Things (IoT) is transforming everyday life.
Stay inspired by the vast knowledge available on Wikipedia.
TechV1